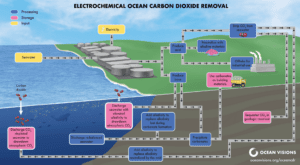
Electrochemical carbon dioxide removal technologies utilize techniques that capture and remove dissolved inorganic carbon from seawater (either as CO2 gas or as calcium carbonate), and/or produce a CO2-reactive chemical base, e.g. sodium hydroxide (NaOH), that can be distributed in the surface ocean to ultimately consume atmospheric CO2 and convert it to long-lived, dissolved, alkaline bicarbonate. These techniques are sometimes called “direct ocean capture” to draw comparisons with direct air capture and encompass both electrodialytic and electrolytic processes.
In electrodialytic approaches, electricity provides the energy to rearrange the most common components of seawater, H2O and NaCl, into acidic (hydrochloric acid; HCl) and basic (sodium hydroxide; NaOH) solutions (House et al., 2007). The acidic solution can then be used to strip dissolved inorganic carbon from seawater in the form of gaseous CO2, or the basic solution can be used to strip the dissolved inorganic carbon by precipitating solid calcium carbonate (Note: Production of calcium carbonate via electrodialysis results in the basic component becoming acidified. This acidic stream may need to be neutralized through addition of a base before being recombined). In addition, the basic component can be selectively added to seawater to draw additional CO2 into the ocean to be stably stored as bicarbonate ions. Electrolytic approaches split water and salt into hydrogen and oxygen and/or chlorine gases, and produce alkaline metal hydroxide (e.g., sodium hydroxide) and acid as byproducts (Rau et al., 2018). When added to surface seawater, the hydroxide reacts with CO2 in seawater to form dissolved alkaline bicarbonate. The resulting CO2 undersaturation in surface seawater then forces atmospheric CO2 to enter the ocean and be stored largely as long-lived seawater bicarbonate. [post_title] => Overview [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => overview [to_ping] => [pinged] => [post_modified] => 2024-09-18 22:30:19 [post_modified_gmt] => 2024-09-18 22:30:19 [post_content_filtered] => [post_parent] => 1909 [guid] => https://oceanvisions.org/roadmaps/electrochemical-cdr/state-of-technology-electrochemical%e2%80%a8-cdr/overview/ [menu_order] => 0 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )Electrodialytic approaches are already commercial technologies used in applications such as whey demineralization, organic acids recovery, and desalination, among others (Bazinet & Geoffroy, 2020).
The electrolytic production of H2, O2/Cl2, hydroxide, and acid is a very mature technology that globally produces some 75 Mt of NaOH per year that is an essential reagent in a variety of important industrial processes (e.g., in food processing, soap production, pulp and paper, pharmaceuticals, etc.) (Lakshmanan & Murugesan, 2014).
An electrolytic approach to precipitate calcium carbonate has recently been proposed (Callagon La Plante et al., 2021). This approach electrochemically generates alkalinity in order to precipitate calcium carbonate, generating a potentially useful end product from the captured carbon. But, as described above, precipitation of calcium carbonate from seawater acidifies the seawater, reducing the ability of seawater to absorb CO2 from air. This proposed pathway is at a technology readiness level of ~5 and is currently undergoing small-scale field testing.
[post_title] => Technology Readiness [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => technology-readiness [to_ping] => [pinged] => [post_modified] => 2024-04-18 23:14:07 [post_modified_gmt] => 2024-04-18 23:14:07 [post_content_filtered] => [post_parent] => 1909 [guid] => https://oceanvisions.org/roadmaps/electrochemical-cdr/state-of-technology-electrochemical%e2%80%a8-cdr/technology-readiness/ [menu_order] => 1 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )- Scalability - Given the immense stocks of dissolved inorganic carbon in seawater (~38,000 Gt (Ciais et al., 2013)), the theoretical scale of carbon capture from electrochemical methods is limitless for all practical intents and purposes. However, engineering, economic, political, and social considerations are likely to significantly reduce this upper bound. These include, but are not limited to:
-
- Access to low or zero-carbon emissions energy and infrastructure (e.g., desalination plants, offshore wind) with the ability to pump large quantities of seawater into large-scale electrochemical reaction cells, perform electrodialysis or electrolysis, and capture outputs and byproducts. The exact energy and infrastructure needs are pathway-dependent. For instance, electrolysis requires more energy than does electrodialysis, but the relative value of beneficial products (e.g., H2, O2 in electrolysis, HCl in electrodialysis) produced by each process must also be considered (Campione et al., 2018).
- In the cases where electrodialysis is used to produce HCl and strip CO2 gas out of seawater or used to produce NaOH that precipitates calcium carbonate, the cost is high (>$350/ton CO2) because of the cost of pumping seawater (Eisaman et al., 2018), and in the case of CO2 stripping, the additional cost of safely storing or utilizing the captured CO2 (Eisaman, 2020)
- Costs can be lowered to ~$100/ton CO2 if captured CO2 is stored in the ocean as bicarbonate due to the elimination of seawater pumping costs{{5}}
- Electrolytic pathways may offer CDR for $150-100/ton CO2, depending upon whether revenues generated from co-products are used to offset gross costs of CO2 capture (Callagon La Plante et al., 2021).
- The collective market size for a suite of co-products and services offered, including concentrated CO2, H2, O2, HCl, control over critical seawater chemistry parameters like pH and total alkalinity, and CDR.
- Access to low or zero-carbon emissions energy and infrastructure (e.g., desalination plants, offshore wind) with the ability to pump large quantities of seawater into large-scale electrochemical reaction cells, perform electrodialysis or electrolysis, and capture outputs and byproducts. The exact energy and infrastructure needs are pathway-dependent. For instance, electrolysis requires more energy than does electrodialysis, but the relative value of beneficial products (e.g., H2, O2 in electrolysis, HCl in electrodialysis) produced by each process must also be considered (Campione et al., 2018).
Ranges from recent synthesis reports vary with the 2021 NASEM report suggesting a range of 0.1-1.0 GtCO2/yr with medium confidence, while the 2023 synthesis report from NOAA suggests 1-10 GtCO2/yr.
- Sequestration Permanence - Electrochemical methods that produce reactive alkaline minerals (e.g., NaOH) will result in additional CO2 from the atmosphere being sequestered in the ocean as bicarbonate ions, which cannot exchange with the atmosphere. This is the same process that results in CO2 sequestration for rock-based forms of ocean alkalinity enhancement. The residence time of bicarbonate ions in the ocean is ~10,000 years, suggesting that electrochemical alkalinity production will generate CO2 sequestration with permanence of ~10,000 years.
Electrochemical methods that produce carbonate mineral products (e.g., Callagon La Plante et al., 2021) with sequestered carbon are stable on timescales of hundreds to thousands of years.
Electrochemical methods that capture and remove CO2 gas from seawater have variable permanence depending upon the storage location for the captured CO2. If the captured CO2 is sequestered in geologic storage, permanence can be long (thousands to millions of years)
[post_title] => CDR Potential [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => cdr-potential [to_ping] => [pinged] => [post_modified] => 2024-04-18 23:20:30 [post_modified_gmt] => 2024-04-18 23:20:30 [post_content_filtered] => [post_parent] => 1909 [guid] => https://oceanvisions.org/roadmaps/electrochemical-cdr/state-of-technology-electrochemical%e2%80%a8-cdr/cdr-potential/ [menu_order] => 2 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )- Electrochemical CDR would likely provide localized reductions in ocean acidification, with expected benefit(s) to marine ecosystems.
- Carbon-negative byproducts from electrochemical CDR can be substituted for more C-intensive sources (Rau, 2008):
- Electrolysis: hydrogen gas, chlorine gas, and oxygen gas, as well as hydrochloric acid
- Electrodialysis: hydrochloric acid
- In comparison to rock-based forms of alkalinity enhancement, electrochemical methods may offer:
- Reduced risk of toxicity from metals present in rock-based forms of alkalinity addition (Hartmann et al., 2013).
- However, the purity of outputs from electrochemical CDR is determined partially by the purity of the input materials to the electrochemical processes, and purer input materials are more expensive.
- Unlike ocean liming and coastal enhanced weathering, electrochemical alkalinity additions will not increase the silica concentration in the ocean and, therefore, may offer a reduced risk of disturbing phytoplankton community dynamics between calcifiers and silicifiers (Bach et al., 2019).
- Risks Shared by Electrolysis and Electrodialysis
- The potential for changes in water column particle concentrations, turbidity, and optical properties if precipitation (inorganic mineral formation) of carbonates occurs due to local increases in alkalinity and pH.
- Mortality of marine life through seawater intake pumps and filters
- Shifts in phytoplankton, invertebrate and vertebrate physiology, competition and/or mortality due to decreases in acidity as CO2 is removed and/or alkalinity is added (Renforth & Henderson, 2017). For example, do the preceding seawater chemistry changes provide an increased competitive advantage for calcifiers over and above the restoration of calcifiers/calcification from ongoing ocean acidification?
- Risks about permanence and impacts of elevated concentrations of bicarbonate in the oceans.
- Risks Specific to Electrolysis
- Chlorine gas production and handling
- Risks Specific to Electrodialysis
- Production of large quantities of acid via electrodialysis that must be safely consumed or neutralized (e.g., via reaction with alkaline minerals).
- For pathways that strip CO2 gas from seawater and sequester the CO2, risks of permanence of storage and potential for leakage
- For pathways that remove dissolved inorganic carbon from seawater as calcium carbonate (Callagon La Plante et al., 2021), the impacts of large quantities of new calcium carbonate on the diversity, abundance, and ecosystem function of marine environments
- Localized reductions in ocean acidification, with expected benefit(s) to industries that rely on these localized improvements such as aquaculture and tourism
- Creation of employment in industries producing carbon-negative byproducts from electrochemical CDR (Rau, 2008):
- Electrolysis: hydrogen gas, chlorine gas, and oxygen gas, as well as hydrochloric acid
- Electrodialysis: hydrochloric acid
- Electrochemical technologies, as applied to mCDR, are very new. Because most field experiments are in the planning phases or are underway currently, it is not yet possible to have a comprehensive characterization of benefits, risks, and scaling considerations of electrochemical approaches in real-world settings (i.e. not benchtop). Controlled field experiments across diverse ecosystems to determine marine chemistry and biology impacts and feedbacks are needed (Develop New Modeling Tools to Support Design and Evaluation, Accelerate Design and Permitting of Controlled Field Trials).
- It is challenging to verify additional CO2 uptake from the atmosphere as a result of electrochemical CDR given the ocean’s dynamic CO2 flux “background state”. New methodologies are needed to observe additional sequestration from the atmosphere into the ocean (Develop New Modeling Tools to Support Design and Evaluation, Develop New In-Water Tools for Autonomous CDR Operations)
- Laboratory experiments are needed across a range of seawater chemistries because of expected electrochemical CDR variations in seawater total alkalinity and dissolved inorganic carbon{{1}} to characterize environmental impacts (Accelerate Design and Permitting of Controlled Field Trials).
- Look to the ocean acidification community’s effort to develop standardized protocols for guidance (Gattuso et al., 2015) as the community builds out standardized protocols and treatment levels for consistency and inter-comparability. See the 2023 Guide to Best Practices in Ocean Alkalinity Enhancement as one example (note that while not all electrochemistry is OAE, there is much in common and there are overlapping needs).
- Global, local, and regional predictions of physical, chemical, and biological outcomes and feedbacks of electrochemical CDR from high-resolution models (Develop New Modeling Tools to Support Design and Evaluation)
- Life cycle assessments to calculate net CDR benefits, taking into account all emissions associated with supply chains (Develop CDR Monitoring and Verification Protocols).
- Current observational technologies (sensors, ROVs, AUVs, etc.) and modeling tools are not widespread and easily available to support field trials with the necessary spatial and temporal frequency of monitoring and sampling (Develop New In-Water Tools for Autonomous CDR Operations)
- In the case of surface seawater alkalinity addition from electrolysis or electrodialysis, cost-effective and safe methods need to be developed that optimize the distribution, dispersal and dilution of any strong chemical bases to avoid impacts of excessively alkaline (pH>9) waters on marine ecosystems. Dilution may require pumping large amounts of seawater, which need to be optimized for energy and cost (Develop New In-Water Tools for Autonomous CDR Operations).
- Offshore deployment of either electrodialysis or electrolysis would require further development to overcome challenges of working in the ocean (corrosion, biofouling, storms, physical and chemical stress on electrodes, catalysts, and membranes, etc.) (Develop New In-Water Tools for Autonomous CDR Operations).
- Questions remain about how variations in seawater particulate and dissolved organic matter, temperature, and salinity affect electrochemical CDR efficiency (Develop New In-Water Tools for Autonomous CDR Operations).
-
- Methods of handling chlorine gas produced as a byproduct of seawater electrolysis are needed (Develop New In-Water Tools for Autonomous CDR Operations, Improve Understanding of Markets for Co-Products)
- Development of feedback control systems that integrate nearby observational data to determine optimal levels of electrochemical CDR (Develop New Modeling Tools to Support Design and Evaluation, Develop New In-Water Tools for Autonomous CDR Operations).
- Identifying and understanding how equipment, energy, and cost scale from benchtop experiments to small field experiments to globally-relevant deployments (Develop New Modeling Tools to Support Design and Evaluation, Develop New In-Water Tools for Autonomous CDR Operations, Develop CDR Monitoring and Verification Protocols, Accelerate RD&D Through New Partnerships)
-
Many of the opportunities and challenges around building public support are not specific to electrochemical CDR, but there are several points of interest specific to electrochemical CDR:
- Electrochemical CDR faces challenges in terms of public perception regarding potential environmental risks not necessarily faced by more “nature-based” approaches, such as coastal blue carbon restoration (Bertram & Merk, 2020). (Accelerate RD&D Through New Partnerships)
- Complexities of electrochemical CDR pathways inhibit understanding and evaluation by non-technical audiences
- There is a great deal of uncertainty and confusion around any mCDR pathway that relies on adding materials to the ocean. This point applies to both electrochemical alkalinity additions, which return a base to the seawater, and pathways that precipitate out calcium carbonate.
- Earlier ocean iron fertilization experiments{{2}} could offer opportunities to adopt best practices and avoid mistakes made when building public support for electrochemical CDR.
- Electrochemical CDR has the advantage that it can be “turned off” more easily than the other approaches, enabling great control over its application
- Clearer communication strategies need to be developed to respond to the “engineering” narrative of electrochemical CDR.
High-resolution data-assimilative models that can support real-world testing of electrochemical CDR are required. These modeling tools must:
- Account for complex interactions in the immediate vicinity of the electrochemical CDR and downstream impacts
- Provide four-dimensional (space and time) estimates of biogeochemistry in zone of influence both in the presence and absence of OAE. The difference between these two simulations can be used to inform CDR estimates that account for background variability in the ocean.
- CDR estimates from electrochemical CDR must include estimates of the “opportunity cost” of electrochemical CDR - how did electrochemical CDR shift phytoplankton community composition, production, and export?
To support the design of proof-of-concept field trials, these models should also:
- Provide estimates of the size and scale of biogeochemical modification to the ecosystem from electrochemical CDR, allowing for informed placement of sensors to monitor the field trials
- Be capable of simulating passive tracers (e.g. SF6) to inform whether and how these passive tracers may be useful in field trials (e.g., estimating rates of atmospheric CO2 uptake)
- Inform a prioritized set of predictions to be tested during field trials
Field trials for the various electrochemical CDR technologies are urgently needed to test both carbon sequestration potential and environmental impacts (both positive and negative). See the mCDR Field Trial Database for information on current field experiments. A series of steps are needed to get to a series of controlled field trials. They include:
[post_title] => Accelerate Design and Permitting of Controlled Field Trials [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => accelerate-design-and-permitting-of-controlled-field-trials [to_ping] => [pinged] => [post_modified] => 2024-04-18 20:09:58 [post_modified_gmt] => 2024-04-18 20:09:58 [post_content_filtered] => [post_parent] => 1911 [guid] => https://oceanvisions.org/accelerate-design-and-permitting-of-controlled-field-trials/ [menu_order] => 1 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )A new suite of durable, seagoing technologies are needed to support electrochemical CDR RD&D. Technology development needs include:
[post_title] => Develop New In-Water Tools for Autonomous CDR Operations [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => develop-new-in-water-tools-for-autonomous-cdr-operations [to_ping] => [pinged] => [post_modified] => 2024-04-18 23:32:35 [post_modified_gmt] => 2024-04-18 23:32:35 [post_content_filtered] => [post_parent] => 1911 [guid] => https://oceanvisions.org/develop-new-in-water-tools-for-autonomous-cdr-operations/ [menu_order] => 2 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )Standardized methodologies from third parties to verify uptake of atmospheric CO2 resulting from electrochemical CDR will ultimately need to be developed to enable trading of carbon removal credits. Key first steps to support development of these protocols include:
- Convening experts to review advances from modeling tools (Develop New Modeling Tools to Support Design and Evaluation) and controlled field trials (Accelerate Design and Permitting of Controlled Field Trials) to identify satisfied and outstanding data needs necessary to quantify additional CO2 uptake as a direct result of electrochemical CDR. As advances in electrochemical CDR RD&D are made, the satisfied and outstanding data needs will need to be updated.
- Apply existing (Koornneed & Nieuwlaar, 2009; Hartmann et al., 2013), or develop when necessary, life cycle analysis tools to calculate stored carbon after accounting for emissions from required materials, energy, transportation/dispersal, etc.
- Include aspects of sustained monitoring to verify CDR permanence over long time scales as CDR is scaled.
Research, development, and demonstration of electrochemical CDR may be accelerated and strengthened by creating partnerships with key industries/sectors, including:
- Offshore renewable energy production, including wind and others, both as power sources and as integrated CDR platforms
- Coastal industries, including desalination and wastewater treatment facilities, which already have infrastructure for pumping/processing seawater or wastewater for CO2 extraction or alkalinity addition.
- Marine research laboratories that already pump seawater and have expertise, technical equipment and infrastructure to support research and development
- Decommissioned or active offshore oil platforms, wells, and reservoirs as sites for CDR and CO2 sequestration.
- Finfish and shellfish aquaculture where the CDR-produced CO2 and/or alkalinity can be used to optimize chemical conditions, including providing relief from ocean acidification.
Developing and strengthening relationships with partner industries may also help promote public acceptance, as well as potentially offer faster routes to obtaining the necessary permitting.
[post_title] => Accelerate RD&D Through New Partnerships [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => accelerate-rdd-through-new-partnerships [to_ping] => [pinged] => [post_modified] => 2023-08-08 16:29:53 [post_modified_gmt] => 2023-08-08 16:29:53 [post_content_filtered] => [post_parent] => 1911 [guid] => https://oceanvisions.org/accelerate-rdd-through-new-partnerships/ [menu_order] => 4 [post_type] => page [post_mime_type] => [comment_count] => 0 [filter] => raw )Electrochemical CDR may generate a host of co-products. Assessing and mapping potential new markets to accommodate these co-products is an important step towards determining the feasibility of electrochemical CDR approaches at scale.
Potential co-products include:
- Hydrogen (H2) gas – as a fuel, energy storage medium, and as a feedstock
- Hydrochloric Acid (HCl) – potentially to refine waste products from silicate mining (House et al., 2007)
- Oxygen (O2) gas
- Chlorine (Cl2) gas – can be combusted with H2 gas to form HCl
Electrochemical CDR
Electrochemical techniques to increase ocean CO2 capture